Introduction: Understanding the Hidden Burden of Osteoporosis Microfractures
Osteoporosis is a silent but progressive bone disease that weakens the skeletal framework, making bones fragile and more prone to tiny, often unnoticed fractures. These microfractures, though small, can accumulate over time, leading to pain, reduced mobility, and a higher risk of major fractures. As the global population ages, osteoporosis has become a major public health issue, affecting both quality of life and healthcare systems. Understanding the underlying mechanisms and exploring new, effective treatments are essential to improving bone strength and long-term recovery outcomes.
The Global Impact of Osteoporosis and Silent Microfractures
Osteoporosis currently affects over 200 million people worldwide, causing millions of fractures each year. These fractures often arise from unnoticed microcracks that progressively weaken bone tissue. Commonly occurring in the spine, hips, and wrists, microfractures can lead to chronic discomfort, loss of height, and reduced independence. Despite their prevalence, these “silent fractures” frequently remain untreated until a major fracture occurs. Beyond the human suffering, osteoporosis-related injuries impose enormous economic burdens, costing healthcare systems billions annually. The growing impact emphasizes the urgent need for preventive care, early diagnosis, and new regenerative therapies capable of restoring bone strength and preventing further microdamage.
Why Osteoporotic Microfractures Often Go Undiagnosed
Osteoporotic microfractures frequently go unnoticed because their symptoms are subtle and nonspecific. Patients often attribute mild back or joint pain to aging or fatigue. Unlike major fractures, microfractures do not always cause visible deformities or acute trauma, making them difficult to detect with conventional X-rays. Advanced imaging tools like MRI or high-resolution CT are more sensitive but not routinely used. Furthermore, general practitioners may focus on pain relief rather than structural bone damage. This diagnostic gap allows microcracks to accumulate, silently degrading bone strength over time. Raising awareness among patients and clinicians about early bone health screening is essential to reduce future fracture risk.
Challenges in Healing Fragile Bones
The healing of osteoporotic bone is inherently slower and less complete than in healthy individuals. Reduced bone mineral density (BMD) limits the mechanical framework needed for repair, while compromised blood circulation delays oxygen and nutrient delivery to damaged areas. The imbalance between osteoblasts (bone-building cells) and osteoclasts (bone-resorbing cells) further impairs tissue regeneration. Many standard treatments, including calcium and bisphosphonate therapy, primarily focus on slowing bone loss rather than promoting new bone formation. As a result, recovery remains incomplete, and patients remain at high risk for recurring fractures. These limitations highlight the need for noninvasive regenerative therapies that can enhance healing and restore skeletal resilience.
The Science Behind Osteoporosis and Bone Microfractures
Osteoporosis is more than a simple loss of bone mass—it is a complex, multifactorial disorder involving cellular imbalance, hormonal changes, and microstructural damage. At the microscopic level, bones become porous and brittle as the natural process of remodeling shifts toward resorption rather than formation. Understanding these biological mechanisms provides a foundation for advanced therapeutic interventions aimed at restoring balance and improving bone quality, not just density. This scientific insight is critical for developing targeted treatments like shockwave therapy.
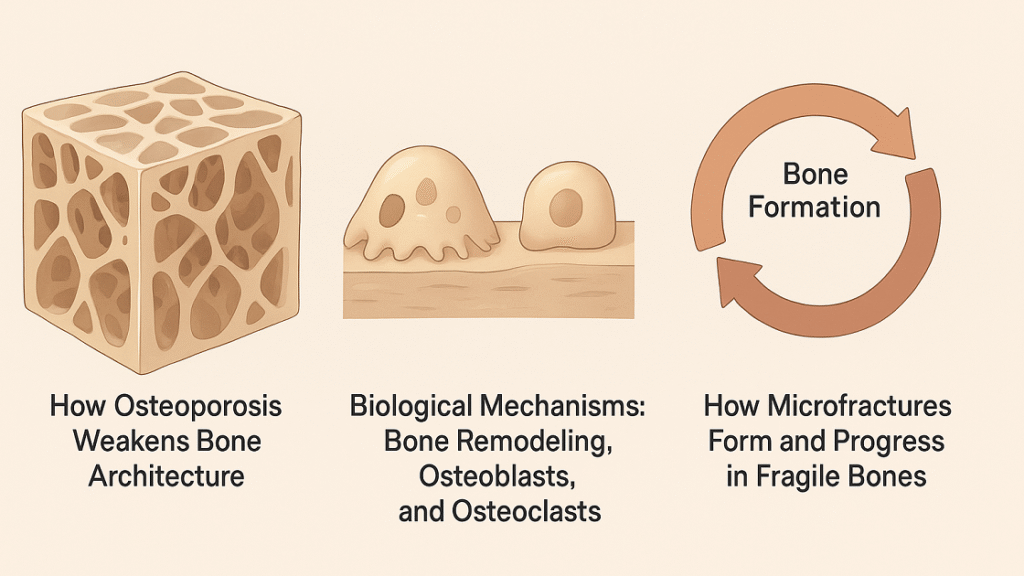
How Osteoporosis Weakens Bone Architecture
Bone is a living tissue composed of dense cortical bone and a porous inner network called trabecular bone. In osteoporosis, trabecular connections thin and break, while cortical bone loses its compact structure, leading to increased porosity and fragility. These structural weaknesses reduce the bone’s ability to absorb normal mechanical stress, causing microscopic cracks even during routine movements. Additionally, lower collagen quality and impaired mineralization diminish elasticity, further predisposing bones to injury. Over time, this deterioration alters bone geometry and mechanical performance, increasing susceptibility to collapse or vertebral compression fractures. Such progressive architectural decline underscores why bone strength cannot be measured solely by density.
Biological Mechanisms: Bone Remodeling, Osteoblasts, and Osteoclasts
Healthy bone maintains strength through continuous remodeling—a balanced process of resorption by osteoclasts and formation by osteoblasts. In osteoporosis, this equilibrium shifts toward bone loss. Age-related hormonal decline, especially reduced estrogen levels, accelerates osteoclast activity and suppresses osteoblast function. Nutritional deficiencies in vitamin D, calcium, and magnesium further exacerbate this imbalance. The result is a porous bone matrix unable to regenerate microdamage efficiently. As remodeling slows, old bone tissue persists, becoming brittle and less resilient. Understanding these biological dynamics reveals why therapeutic interventions must not only slow resorption but also reactivate osteoblast-driven bone formation to restore structural integrity.
How Microfractures Form and Progress in Fragile Bones
Microfractures arise when daily mechanical loads exceed the bone’s diminished capacity for repair. In healthy skeletons, minor microdamage is promptly detected and replaced through remodeling. In osteoporotic bones, however, insufficient osteoblast activity, poor vascularization, and disrupted signaling prevent timely healing. The resulting microcracks accumulate, merging into larger defects that compromise mechanical stability. Over time, these injuries can trigger localized inflammation, pain, and vertebral deformities. Without intervention, the progressive cycle of microdamage and insufficient repair culminates in severe fractures. Early detection and stimulative therapies that promote local regeneration are therefore vital to halt this destructive cascade.
Shockwave Therapy in Osteoporosis Treatment
Having established the foundational understanding of both osteoporosis pathophysiology and shockwave therapy principles, we now examine the specific applications and mechanisms through which this technology addresses osteoporotic bone pathology. The convergence of mechanical stimulation and biological bone responses creates unique therapeutic opportunities for patients with compromised skeletal integrity.
How Shockwave Therapy Promotes Bone Healing
Terapia de ondas de choque accelerates bone healing through multifaceted mechanisms targeting both cellular and molecular levels. The mechanical stimulation directly activates osteoprogenitor cells and mature osteoblasts, enhancing their proliferation and differentiation capacity. Shockwaves modulate the bone remodeling cycle by promoting osteoblastic activity while potentially attenuating excessive osteoclastic resorption. The induced controlled microtrauma stimulates the fracture healing cascade without creating additional structural damage. Growth factor upregulation, particularly bone morphogenetic proteins and insulin-like growth factors, creates an osteogenic microenvironment. This combined effect results in accelerated callus formation and enhanced bone matrix mineralization.
Cellular Mechanisms: Osteoblast Activation and Angiogenesis
At the cellular level, shockwaves trigger osteoblast activation through integrin-mediated mechanotransduction pathways, leading to increased alkaline phosphatase activity and osteocalcin synthesis—key markers of bone formation. The therapy stimulates mesenchymal stem cell differentiation toward osteoblastic lineages through Wnt/β-catenin signaling pathway activation. Simultaneously, robust angiogenic responses occur through VEGF and endothelial nitric oxide synthase (eNOS) upregulation, enhancing blood vessel ingrowth into bone tissue. Improved vascularization facilitates nutrient delivery, oxygenation, and cellular recruitment essential for bone regeneration. These coordinated cellular responses create optimal conditions for microfracture repair.
Enhancing Bone Mineral Density (BMD) through Mechanical Stimulation
Shockwave therapy provides beneficial mechanical loading to osteoporotic bone, operating on Wolff’s Law principles that bone adapts to applied mechanical stress. The controlled mechanical stimulation activates mechanosensitive osteocytes—the bone’s mechanosensors—which orchestrate remodeling responses through the lacunar-canalicular network. This mechanical signaling inhibits sclerostin production by osteocytes, removing a natural brake on osteoblastic bone formation. Studies demonstrate measurable BMD increases in treated regions through enhanced mineralization and bone matrix deposition. The mechanical stimulus also improves bone microarchitecture beyond density alone, optimizing trabecular connectivity and cortical thickness contributing to structural competence.
Shockwave-Induced Pain Reduction and Mobility Improvement
Beyond structural bone benefits, shockwave therapy provides significant symptomatic relief for osteoporotic patients experiencing microfracture-related pain. The mechanism involves hyperstimulation analgesia—overwhelming nociceptive nerve fibers with mechanical stimulation—and substance P depletion, reducing pain signal transmission. Additionally, inflammatory mediator modulation and improved tissue perfusion contribute to analgesic effects. Patients typically experience progressive pain reduction over treatment series, enabling increased physical activity participation. Enhanced mobility creates positive feedback through mechanical loading stimulation of bone remodeling. This dual benefit of structural improvement and symptomatic relief makes ESWT particularly valuable for osteoporotic patient management.
Pruebas clínicas y resultados de la investigación
Transitioning from mechanistic understanding to practical clinical application requires rigorous examination of available evidence supporting shockwave therapy for osteoporosis treatment. The growing body of research encompasses preclinical studies, controlled trials, and clinical observational data that collectively inform evidence-based practice recommendations for this emerging therapeutic modality.
Key Studies Supporting Shockwave Therapy for Osteoporosis
Multiple preclinical and clinical investigations demonstrate shockwave therapy’s efficacy in osteoporotic bone treatment. Animal studies using ovariectomized rat models—the standard experimental osteoporosis model—consistently show improved BMD, enhanced trabecular architecture, and increased bone strength following ESWT. Clinical pilot studies in postmenopausal women with osteoporosis report BMD improvements of 5-12% in treated regions over 6-12 month periods. Research examining vertebral compression fracture treatment demonstrates accelerated healing and reduced pain scores. Studies investigating distal radius fracture healing in osteoporotic patients show shortened union times and improved functional outcomes with adjunctive shockwave therapy.
Clinical Outcomes: Healing Time and Bone Strength
Clinical outcome data indicate shockwave therapy meaningfully impacts both healing kinetics and ultimate bone quality parameters. Fracture healing time reductions of 20-40% have been reported in comparative studies, with earlier radiographic evidence of callus formation and bridging. Biomechanical testing of treated bone demonstrates improved ultimate load capacity, stiffness, and energy absorption compared to control treatments. Quality of life assessments reveal sustained functional improvements and reduced fracture-related disability. Bone turnover marker studies show beneficial shifts with increased formation markers (osteocalcin, bone-specific alkaline phosphatase) and maintained or decreased resorption markers (C-terminal telopeptide), reflecting improved bone balance.
Patient Case Studies and Real-World Applications
Real-world clinical applications provide valuable insights into shockwave therapy’s practical utility for osteoporotic patients. Case reports describe successful treatment of delayed union vertebral compression fractures in elderly patients who achieved pain resolution and radiographic healing after failing conservative management. Clinical series document hip fracture patients receiving adjunctive ESWT demonstrating earlier mobilization and shorter rehabilitation periods. Outpatient case management protocols show feasibility and acceptability, with most patients tolerating treatment well with minimal side effects limited to transient erythema or mild discomfort. Long-term follow-up cases indicate sustained benefits, suggesting durable therapeutic effects beyond immediate treatment periods.
Integrative Approaches for Stronger Bones
Optimal osteoporosis management transcends single-modality treatment, requiring comprehensive strategies that address multiple facets of bone health. While shockwave therapy offers valuable mechanical and biological stimulation, integrating this technology with nutritional optimization, appropriate exercise prescription, and lifestyle modifications creates synergistic effects that maximize therapeutic outcomes and promote long-term skeletal health maintenance.
Combining Shockwave Therapy with Nutrition and Supplements
Shockwave therapy’s bone-building effects are optimized when combined with adequate nutritional substrate for bone formation. The mechanical and biological stimulation provided by ESWT increases bone metabolic demands, requiring sufficient nutrient availability for optimal osteoblastic activity. Coordinating treatment timing with nutritional optimization ensures that the upregulated bone formation signals can translate into actual matrix synthesis and mineralization. Protein intake particularly warrants attention, as collagen synthesis—comprising 90% of bone organic matrix—requires adequate amino acid availability. This integrative approach addresses both the biological signaling (through ESWT) and material substrate (through nutrition) necessary for bone regeneration.
Role of Calcium, Vitamin D, and Magnesium in Bone Health
Calcium represents the principal mineral constituent of hydroxyapatite crystals comprising bone’s inorganic phase, with daily requirements of 1000-1200mg for osteoporotic patients. Vitamin D facilitates intestinal calcium absorption and directly influences osteoblast function through vitamin D receptor activation, with serum 25-hydroxyvitamin D levels optimally maintained above 30ng/mL. Magnesium participates in bone mineralization, influences parathyroid hormone secretion, and affects vitamin D metabolism, with recommended intake of 320-420mg daily. These nutrients work synergistically—vitamin D enhances calcium absorption, while magnesium facilitates vitamin D conversion to active forms. Supplementation should be tailored to individual deficiency status verified through laboratory assessment.
Exercise and Weight-Bearing Activities to Complement ESWT
Resistance training enhances bone strength through mechanical loading.
Weight-bearing exercises like walking and climbing stimulate bone growth.
Regular strength training 2–3 times weekly builds bone and muscle.
Balance exercises reduce fall risk and prevent osteoporosis-related injuries.
Lifestyle Modifications for Long-Term Bone Strength
Stop smoking to restore osteoblast function and bone healing capacity.
Limit alcohol to protect calcium balance and bone metabolism.
Maintain healthy weight to support hormonal balance and bone stability.
Prevent falls with safe home setups, footwear, and vision checks.
Prioritize sleep and stress control to optimize bone-building hormones.
Conclusion: Building Stronger Bones Through Science and Innovation
Extracorporeal Shockwave Therapy (ESWT) represents a major advancement in osteoporosis management, shifting from pharmacological treatment to mechanobiological regeneration. By delivering precise mechanical energy, ESWT stimulates osteoblast activity, angiogenesis, and bone remodeling—strengthening fragile bone architecture. Clinical studies show improved bone mineral density, faster fracture healing, and pain reduction with minimal side effects. Optimal outcomes occur when ESWT is integrated with nutrition rich in calcium, vitamin D, and protein, along with weight-bearing exercise and lifestyle optimization. This holistic approach enhances bone repair, prevents future fractures, and promotes long-term skeletal health. Continued research aims to refine treatment parameters and personalize therapy based on bone quality. Ultimately, ESWT exemplifies how science and innovation can restore strength, mobility, and independence—empowering patients to overcome osteoporosis and maintain quality of life.
Referencias
- Aumento de la densidad ósea: ¿Puede la terapia de ondas de choque ayudar a mi osteoporosis?
- Papel de la terapia de ondas de choque en la curación de fracturas
- Aceleración de la curación de fracturas: El poder de la terapia de ondas de choque
- Slightly focused high-energy shockwave therapy: a potential adjuvant treatment for osteoporotic fracture
- Upregulation of VEGF in Subchondral Bone of Necrotic Femoral Heads in Rabbits with Use of Extracorporeal Shock Waves
